Beyond Cancer® Publishes Pre-Clinical Data in Cancer Cell International Demonstrating Its Tumor Ablation Method Utilizing Ultra-High Concentration Nitric Oxide (UNO) Induced a Systemic Response that Prevents Metastases
December 13, 2022 | 9:26 AM ESTData describe a potent systemic immune response resulting in a significant reduction in challenge tumors and improvement in survival for UNO treated mice
GARDEN CITY, N.Y. and HAMILTON, Bermuda, Dec. 13, 2022 — Beyond Cancer, Ltd., an affiliate of Beyond Air, Inc. (NASDAQ: XAIR) that is focused on developing ultra-high concentration nitric oxide (UNO) for the treatment of solid tumors, announced today the publication of pre-clinical data demonstrating that its proprietary tumor ablation technology utilizing UNO results in a potent innate and adaptive immune response that prevents metastases and resulted in a statistically significant survival benefit.
The data were published in an article entitled, “Gaseous Nitric Oxide Tumor Ablation Induces an Anti-Tumor Abscopal Effect,” in the peer-reviewed journal Cancer Cell International (“CCI”). The article is available at (click here).
“Beyond Cancer’s novel implementation of ultra-high concentrations of nitric oxide to treat solid tumors has repeatedly resulted in data demonstrating an immunostimulatory response,” stated Dr. Hila Confino, Chief Scientific Officer. “By locally ablating tumors with UNO we have shown that the recruitment of antigen-presenting cells (APCs) triggers T-cells to proliferate and mature, producing a long-term systemic immune response that reduces distant metastases and prolongs survival in mice.”
Dr. Frederick Dirbas, MD, Associate Professor of Surgery, Div of Surgical Oncology, Dept of Surgery, Stanford University School of Medicine commented, “Beyond Cancer’s UNO technology data in the published manuscript demonstrates promising immunotherapeutic responses with the possibility of being a novel treatment for solid tumors.”
The manuscript published in CCI describes several studies of in-vitro and in-situ tumor ablation utilizing a single intratumoral treatment of UNO with secondary anti-tumor effects. In one study, CT26 tumor-bearing mice were treated with a single 5-minute administration of 50,000 ppm gaseous nitric oxide (gNO) or 20,000 ppm gNO versus naïve control. Two weeks post gas treatment, the tumors were resected and a week later a secondary tumor was induced in the contralateral flank. As shown in the figure below at day 55, all of the mice treated with 50,000 ppm gNO rejected the secondary CT26 cell inoculation versus none of the mice in the naïve group. In addition, 73% of the mice treated with 20,000 ppm gNO rejected the secondary inoculation, also a significantly better result than naïve control. By Day 75, the conclusion of the study, 89% of the mice treated with 50,000 ppm gNO and 64% of the mice treated with 20,000 ppm gNO rejected a secondary inoculation, compared to none of the mice in the naïve group.
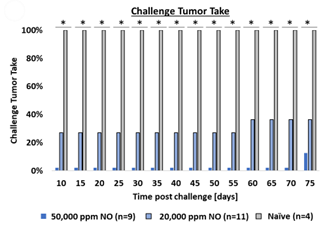
Caption: Challenge tumor take was markedly reduced in UNO treated mice vs the naïve control group
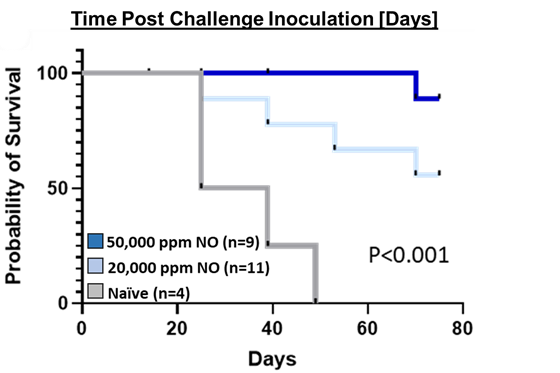
Caption: A significant survival advantage (P < 0.001) was achieved in UNO treated mice compared to control
The Kaplan-Meier analysis in the figure above shows statistical significance for survival in UNO treated CT26 tumor-bearing mice versus control. There was also a separation between the 50,000 ppm arm and the 20,000 ppm arm implying a potential dose response.
“These impressive data published in the peer-reviewed journal CCI support our understanding of UNO as an immunomodulating therapeutic option for the treatment of solid tumors,” said Dr. Selena Chaisson, Chief Executive Officer. “Throughout the year, the team at Beyond Cancer has made incredible progress translating the entire preclinical effort for UNO into the clinic via the first-in-human Phase 1 trial. We look forward to further elucidating the powerful effects of UNO on human tumors.”
About Nitric Oxide (NO)
Nitric Oxide (NO) is a powerful molecule, naturally synthesized in the human body, proven to play a critical role in a broad array of biological functions. Currently, exogenous inhaled NO is used in adult respiratory distress syndrome, post certain cardiac surgeries, and persistent pulmonary hypertension of the newborn to treat hypoxemia. Additionally, NO is believed to play a key role in the innate and adaptive immune system response and in vitro studies suggest that NO possesses broad-spectrum antimicrobial activity and anticancer properties.
About Beyond Cancer, Ltd.
Beyond Cancer, Ltd., an affiliate of Beyond Air, Inc., is a development-stage biopharmaceutical and medical device company utilizing ultra-high concentration nitric oxide (UNO) via a proprietary delivery platform to treat primary tumors and prevent metastatic disease. Nitric oxide at ultra-high concentrations has been reported to show anticancer properties and to potentially serve as a chemosensitizer and radiotherapy enhancer. A first-in-human study is underway in patients with solid tumors. The Company is conducting preclinical studies of UNO in multiple solid tumor models to inform additional treatment protocols.
For more information, visit www.beyondcancer.com.
About UNO Therapy for Solid Tumors
Cancer is the second leading cause of death globally, with tumor metastases responsible for approximately 90% of all cancer-related deaths. Current cancer treatment modalities generally include chemotherapy, immunotherapy, radiation, and/or surgery. Ultra-high concentration Nitric Oxide (UNO) therapy is a completely new approach to preventing relapse or metastatic disease. In vitro murine data show that local tumor ablation with UNO stimulates an anti-tumor immune response in solid tumor cancer models. The Company believes that UNO has the potential to prevent relapse or metastatic disease with as little as a single 5-minute treatment and with limited toxicity or off-target effects.
About Beyond Air, Inc.
Beyond Air, Inc. (Beyond Air) (NASDAQ: XAIR) is a medical device and biopharmaceutical company dedicated to harnessing the power of nitric oxide (NO) through its revolutionary NO Generator and Delivery System, LungFit®, that uses NO generated from ambient air to deliver precise amounts of NO to the lungs for the potential treatment of a variety of pulmonary diseases. The LungFit can generate up to 400 ppm of NO, for delivery either continuously or for a fixed amount of time and has the ability to either titrate dose on demand or maintain a constant dose. Beyond Air has received FDA approval for its first system, LungFit PH to treat term and near-term neonates with hypoxic respiratory failure. Beyond Air is currently advancing its other revolutionary LungFit systems in clinical trials for the treatment of severe lung infections such as viral community-acquired pneumonia (including COVID-19), nontuberculous mycobacteria (NTM) and severe lung infections in other settings.
For more information, visit www.beyondair.net.
Forward Looking Statements
This press release contains “forward-looking statements” concerning the potential safety and efficacy of inhale nitric oxide and the ultra-high concentration nitric oxide product candidate, as well as its therapeutic potential in a number of indications; and the potential impact on patients and anticipated benefits associated with inhaled nitric oxide and the ultra-high concentration nitric oxide product candidate. Forward-looking statements include statements about expectations, beliefs, or intentions regarding product offerings, business, results of operations, strategies or prospects. You can identify such forward-looking statements by the words “expects,” “plans,” “anticipates,” “believes” “expects,” “intends,” “looks,” “projects,” “goal,” “assumes,” “targets” and similar expressions and/or the use of future tense or conditional constructions (such as “will,” “may,” “could,” “should” and the like) and by the fact that these statements do not relate strictly to historical or current matters. Rather, forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause actual results to differ materially from any future results expressed or implied by the forward-looking statements. These forward-looking statements are only predictions and reflect views as of the date they are made with respect to future events and financial performance. Many factors could cause actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including risks related to: Beyond Cancer’s ability to raise additional capital; the timing and results of future pre-clinical studies and clinical trials concerning the ultra-high concentration nitric oxide product candidate; the potential that regulatory authorities, including the FDA and comparable non-U.S. regulatory authorities, may not grant or may delay approval for the ultra-high concentration nitric oxide product candidate; the approach to discover and develop novel drugs, which is unproven and may never lead to efficacious or marketable products; Beyond Cancer’s ability to fund and the results of further pre-clinical studies and clinical trials of the ultra-high concentration nitric oxide product candidate; obtaining, maintaining and protecting intellectual property utilized by products; obtaining regulatory approval for products; competition from others using similar technology and others developing products for similar uses; dependence on collaborators; and other risks, which may, in part, be identified and described in the “Risk Factors” section of Beyond Air’s most recent Annual Report on Form 10-K and other of its filings with the Securities and Exchange Commission, all of which are available on Beyond Air’s website. Beyond Cancer and Beyond Air undertake no obligation to update, and have no policy of updating or revising, these forward-looking statements, except as required by applicable law.
CONTACTS:
Corey Davis, Ph.D.
LifeSci Advisors, LLC
Cdavis@lifesciadvisors.com
(212) 915-2577
Matt Johnson, Head of Corporate Development & Strategy
Beyond Cancer, Ltd.
Mjohnson@beyondcancer.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/26d99aea-0c22-4ac8-81fb-413d504afdfb
https://www.globenewswire.com/NewsRoom/AttachmentNg/95e1d260-011a-4fd6-b8f4-93dcb36b34c1
